Properties of Pure Substance
- A pure substance is a material with homogeneous and invariable composition.
- Pure substances can have multiple phases, an ice water mixture is still a pure substance but an air-steam is not a pure substance.
Pure Substance:
- A substance that has a fixed chemical composition throughout is called a pure substance i.e., water, nitrogen, helium, and CO2.
- Substances which are mixture of various elements or compounds also qualifies as pure substances as long as mixture is homogeneous.

Saturation Temperature and Saturation Pressure:
- At given pressure, the temperature at which a pure substance changes phase is called the saturation temperature Tsat.
- Likewise at a given temperature, the pressure at which a pure substance changes phase is called the saturation pressure psat.
Example: For water at a pressure of 101.325 kPa, Tsat is 100oC, conversely at a temperature of 100oC, psat is 101.325 kPa.
Latent Heat:
- The amount of energy absorbed or released during a phase change process is called the latent heat.
- The amount of energy absorbed during melting is called the latent heat of vaporization.
- Similarly, the amount of energy absorbed during vaporization is called latent heat of vaporization and is equivalent to the energy released during condensation.
Liquid-Vapour Saturation Curve:
From the following figure, it is clear that Tsat increases with psat. Thus, a substance at higher pressure will boil at higher temperatures.
Tsat = f(psat)

- In the kitchen, higher boiling temperature means shorter cooking time and energy saving.
- The atmospheric pressure, and thus the boiling temperature of water, decreases with elevation. Therefore, it takes longer time to cook at higher altitudes than it does at sea level.
Property Diagrams for Phase-change Process
The T-V Diagram:
- Consider piston cylinder device containing liquid water at 20oC and 1 atm.
- Water will start boiling at a much higher temperature (179.9oC) at inside pressure of the cylinder reaches at 1 MP.
- The specific volume of the saturated liquid is larger and the specific volume of the saturated vapour is smaller than the corresponding values at 1 atm pressure. That is, the horizontal line that connects the saturated liquid and saturated vapour states is much shorter.
- As the pressure is increased further, this saturation line will continue to get shorter as shown in figure and it will become a point when the pressure reaches 22.09 MPa for the case of water. This point is called the critical point and it is defined as the point at which the saturated liquid and saturated vapour state are identical.
- At pressure above the critical pressure, there will not be a distinct phase change. Instead, the specific volume of the substance will continually increase and at all times there will be only one phase present. It is customary to refer to the substance as superheated vapour at temperature above the critical temperature and as compressed liquid at temperatures below the critical temperature.

The p-V Diagram:
- The general shape of the p-V diagram of a pure substance is very much like the T-V diagram but the T = constant lines on this diagram have a downward trend.
- Consider again a piston cylinder device that contains liquid water at 1 MPa and 150°C, water at this state exists as a compressed liquid. Now, the weights on top of the piston are removed one by one so that the pressure inside the cylinder decreases gradually.
- The water is allowed to exchange heat with the surroundings so its temperature remains constant.
- As the pressure decreases, the volume of the water will increase slightly, when the pressure reaches the saturation pressure volume at the specific temperature, the water will start to boil.
- During this vaporisation process, both the temperature and the pressure remain constant but the specific volume increases. Once the last drop of liquid is vaporised further reduction in pressure results in a further increase in specific volume.
- If the process is repeated for other temperatures similar paths will be obtained for the phase change processes.
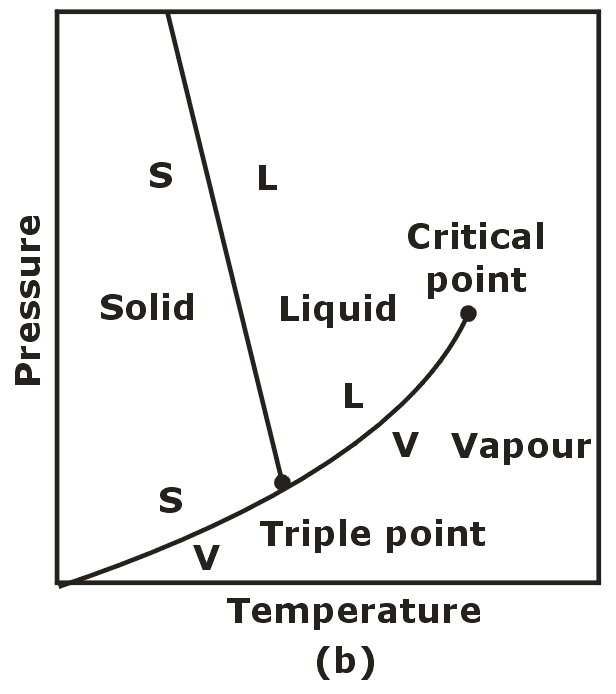
Triple Phase:
- When all three phases of a pure substance co-exist in equilibrium. It is called triple phase.
- Triple phase states form a line called the triple line.
- The triple line appears as a point on the p-T diagram and therefore is often called the triple point.

- No substance can exist in the liquid phase in stable equilibrium at pressure below the triple point pressure.
- The same can be said for temperature for substance that contract on freezing.
- Substances at high pressure can exist in the liquid phase at temperatures below the triple point temperature.
The p-T Diagram:
- Solid – Liquid = Fusion
- Liquid – Vapour = Vaporisation
- Solid – Vapour = Sublimation
Enthalpy
- Enthalpy is a measure of the total energy of a thermodynamic system.
- It includes energy required to create a system and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.
- For certain type of processes particularly in power generation and refrigeration.
- Enthalpy (H )= U + pV(kJ) Or
- per unit mass h = u + pV(kJ/kg)
Saturated Liquid and Saturated Vapour States:
- Subscript f → properties of saturated liquid
- Subscript g → properties of saturated vapour
- Vf → specific volume of saturated liquid
- Vg → specific volume of saturated vapour
- Vfg → difference between Vg and Vfnthal
- Vfg = Vg - Vf
- hfg → enthalpy of vaporisation or latent heat of vaporisation (amount of energy need to vaporise a unit mass of saturated liquid at a given temperature of pressure).
- The magnitude of latent heat depends on temperature and pressure at which phase change occurs.
- For example at 1 atm pressure, the latent heat of fusion of water is 333.7 kj/kg and latent heat of vaporization is 2257.1 kj/kg.
- At below triple point pressure, substance begins to change directly gas.
- Enthalpy of vaporization decreases as the temperature or pressure increases and become zero at the critical point.
Saturated Liquid Vapour Mixture:
- During vaporisation process, a substance exists as part liquid and part vapour.
- A new property quality x is the ratio of mass of vapour to the total mass of the mixture.
Quality or dryness fraction:

 mtotal = mliquid + mvapour = mf + mg
mtotal = mliquid + mvapour = mf + mg
where, mf = mass of the saturated liquid, and mg = mass of the saturated vapour
- Quality has significance for saturated mixtures only.
- It has no meaning in the compressed liquid or superheated region.
- Its value lie between 0 (saturated liquid) and 1 (saturated vapour).
- The properties of the saturated liquid are the same whether it exists alone or in a mixture with saturated vapour.
- During vaporization process, only the amount of saturated liquid changes not its properties. The same can be said about a saturated vapour.
Quality :

- Vav = (1-x)Vf + xVg or Vav = Vf + xVg
- Uav = Uf + Ufg
- hav = hf + xhfg
Superheated Vapour :
Since, the superheated region is a single phase region (vapour phase only) temperature and pressure are no longer dependent properties and they can conveniently be used as the two independent properties.
Superheated vapour is characterised by:
- Lower pressures (p < psat at a given T)
- Higher temperatures (T < Tsat at a given p)
- Higher specific volumes (U > Vg at a given p or T)
- Higher internal energies (U > Ug at a given p or T)
- Higher enthalpies (h > hg at a given p or T)
Compressed Liquid
- A compressed liquid may be approximated as a saturated liquid at the given temperature.
- This is because the compressed liquid properties depend on temperature much more strongly than they do on the pressure.
- V ≅ Vf
- u ≅ uf
- h ≅ hf
- In general, a compressed liquid is characterised by:
- Higher pressures (p > psat at a given T)
- Lower temperatures (T < Tsat at a given p)
- Lower specific volumes (V <Vf at a given p or T)
- Lower internal energies (U < Uf at a given p or T)
- Lower enthalpies (h < hf at a given p or T)
The Ideal Gas Equation of State
- Any equation that relates the pressure, temperature and specific volume of a substance is called an equation of state.
- Property relations that involve other properties of a substance at equilibrium states are also referred to as equation of state.
- Ideal gas equation of state: pV = RT, where, p = Absolute pressure, T = Absolute temperature, and V = Specific volume
- Gas and vapour are often used as synonymous words.
- The vapour phase of a substance is customarily called a gas when it is above the critical temperature.
- Vapour usually implies a gas that is not far from a state of condensation.
Gas Constant
- It has been experimentally observed that the ideal gas relation given closely approximately the p-V-T behaviour of real gases at low densities.
- At low pressure and high temperature, the density of a gas decreases and the gas behaves as an ideal gas under these conditions.

where, Ru = Universal gas constant, M = Molar mass, R = Gas constant.
Compressibility Factor
- Compressibility factor (correction factor) is measurement of deviation of gases from ideal gas behaviour.
- Compressibility factor (z):
![]()
- It can also be expressed as

- For ideal gases ⇒ z = 1
- For real gases ⇒ z is away from unity (> 1 or < 1)
Reduce Pressure and Temperature
- Gases behave differently at a given temperature and pressure, but they behave very much the same at temperature and pressures normalized with respect to their critical temperatures and pressures.
- The normalization is done as introducing new terms,
- Reduce pressure:

and reduce temperature:

where, Tcr = critical temperature, pcr = critical pressure.

z factor for all gases is approximately the same at the same reduced pressure and temperature is greatest in the vicinity of the critical point.
- Gases deviate from the ideal gas behaviour most in the neighborhood of the critical point. So, we can say that at critical point. Compressibility factor is constant for all substances.
- As mention above z factor for all gases is approximately the same at the same reduced pressure and temperature.
- Pseudo reduced specific volume (VR) :

- VR is defined differently from pR and TR. It is related to Tcr and pcr instead of Vcr.
- All substances have same critical compressibility factor

- Experimental value of Zc for most substances fall within a narrow range of 0.20 – 0.33.
- Z the compressibility factor is the same function of pr and Tr for all gases.
- Specific heat all constant pressure and volume are the properties of the substances and are always properties.

Van der wall’s Equation of State:

- Two effects: Inter molecular attraction forces:
![]() and b accounts for volume occupied by the gas molecules.
and b accounts for volume occupied by the gas molecules.

Virial Equation of State:
Van der Waal’s equation of state can be expressed in the virial form as given below

This is called the virital equation of state.
No comments:
Post a Comment
Knowing brings controversy