Ideal Fluid
Characteristics of Ideal Fluid
- Ideal fluid should be having zero viscosity and zero surface tension.
- Ideal fluid is incompressible.
- There is no ideal fluid that posses all the properties but air and water are considered as ideal fluid.
Real fluid
Characteristics of real fluid
- Fluid having viscosity and surface tension.
- Real fluids are compressible
Properties of Fluid
Properties of fluid are as follows:
(1). Intensive properties:
- Intensive properties are those properties which do not dependent on mass.
- Example: Temperature, pressure, density, Boiling and Melting point, refractive index, etc.
(2). Extensive properties
- Extensive properties are those properties that are dependent on mass.
- Example – mass, Energy, Volume, Momentum, etc.
Mass Density (ρ):
- It is the mass of fluid per unit volume at a given temperature and pressure.
- Mass density is function of Temperature and Pressure.
- Mass density for gases inversely proportional to temperature & directly proportional to pressure.
Since for an ideal gas:
P =ρRT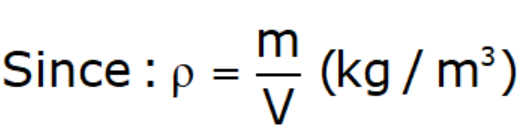
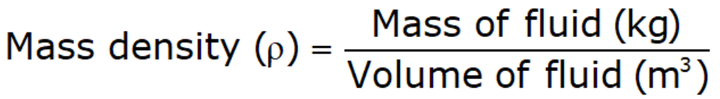
Unit: kg/m3 or g/cc- At 4°C and 1 atm pressure: ρwater = 1000 kg/m3 = 1 g/cc
Specific Weight or Weight Density [w or r]
- Specific density is also known as weight density is defined as the ratio of the weight of the fluid to its volume.
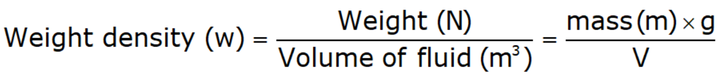
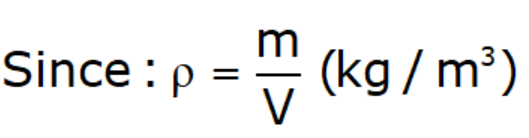
Thus, w = ρg = N/m3
Where: g → acceleration due to gravity. - w for water at 4°C and 1 atm = 1000 × 9.8 N/m3 = 9.8 kN/m3
Specific Volume
- Specific volume is Reciprocal of specific mass.
- The volume of fluid per unit mass
- Unit of specific volume:
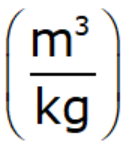
Specific Gravity or Relative Density
- Specific gravity is the ratio of the specific weight of the fluid to the specific weight of the standard fluid.
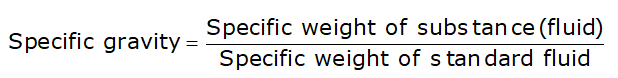
- Standard fluid
- Liquid – water at 4ºC
- Gas – Hydrogen or Air
- Specific gravity is a dimensionless quantity.
- Relative density is the ratio of the density of a fluid to the density of another fluid (not necessarily water).
- Whereas specific gravity is the ratio of the density of a fluid to the density of the standard fluid (i.e., water at 4ºC).
- For taking water as standard fluid:
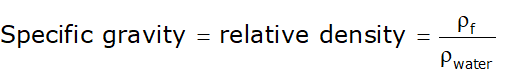
Viscosity
- Viscosity tells about the internal resistance of a fluid to its own flow.
- Viscosity is a measure of the resistance offered by a fluid layer to an adjacent layer of fluid at motion.
- Viscosity is due to the internal friction force caused by cohesive force between fluid molecules (dominant in fluid) and molecular momentum transfer between particles due to collision (dominant in gases).
Assume a system having fluid between two plates.
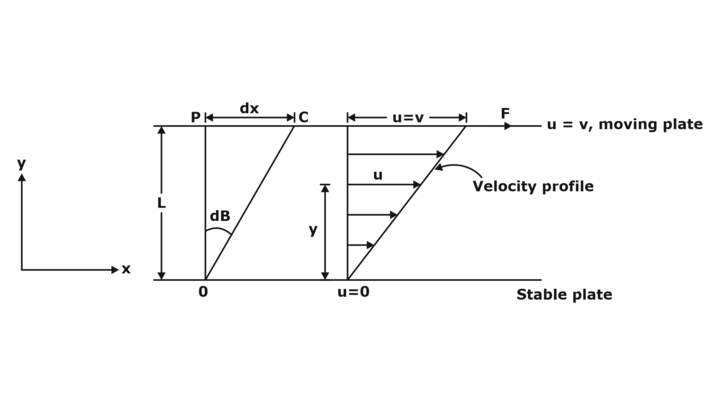
Note:
- Assume linear variation of velocity
- dβ = Angle of deformation during ‘dt’ duration
- From similar triangle:
Velocity at distance y from bottom plate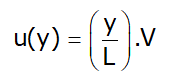
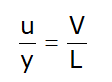
- If consider infinite small element then velocity gradient=
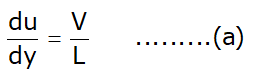
- Displacement of fluid element at p to c during small time interval dt :
dx = Vdt .......... (b)
Put value of dx from equation (b):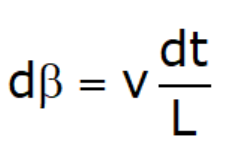
Now replace V from equation (a):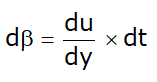
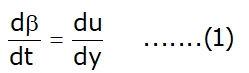
- Angular deformation rate is equal to the velocity gradient.
According to Newton
- Rate of deformation is proportional to shear stress, so:
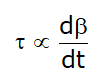
Now, from equation (1):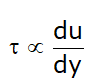
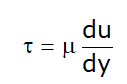
- μ → Absolute viscosity or Dynamic viscosity or Coefficient of viscosity.
- Unit:
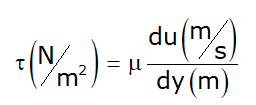
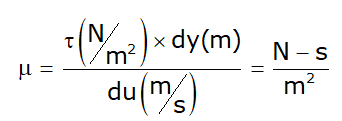
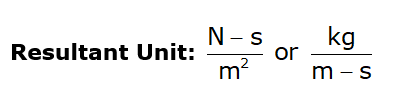
Note: Viscosity of water at 20°C = 1 centipoise - Poise is a CGS unit: poise = Dyne-s/cm2
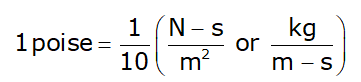
Kinematic Viscosity
- It is the ratio of dynamic viscosity (μ) and density of fluid(ρ).
- Kinematic viscosity denoted by (ν):
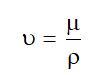
Units:
Classification of Fluid
Classification of fluid as per relation between shear stress and rate of deformation:
Newtonian Fluid:
- Fluid follows Newton’s law of viscosity.
- Example: Water, petrol, diesel, alcohol, all gases etc.
Non-Newtonian Fluid:
- Thixotropic {pseudo–plastic}
- The slope of curve between “shear stress - deformation rate” decreases with increasing in deformation rate.
- Also known as “shear thinning” fluids
- Example: Printer ink
- Dilatant:
- Slope of shear “shear stress Vs deformation rate curve” increases with rate of deformation.
- Example – Quicksand
- Also known as "shear thickening" fluid.
- Ideal Plastic / Bingham Plastic:
- Having an initial yield stress and then exhibit a linear relationship between
- Example: Toothpaste, drilling mud etc.
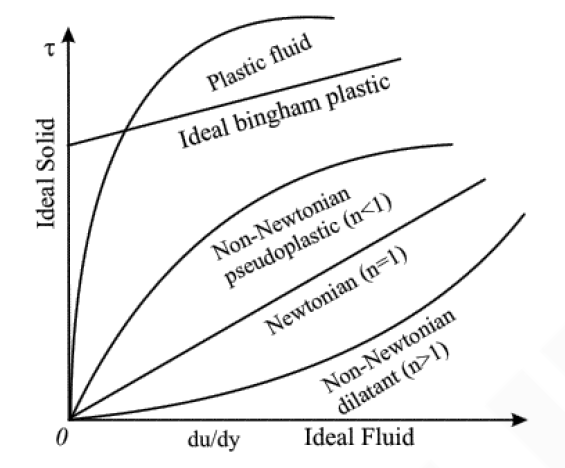
- Having an initial yield stress and then exhibit a linear relationship between
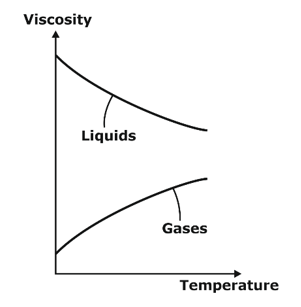
The Dependency of Viscosity on Temperature
For Gases:
- In gases, the Viscosity offered by the fluid is due to molecular momentum transfer.
- With the increase in temperature, molecular momentum transfer of fluid increases so viscosity of gases increases with increase in temperature.
For liquids:
- In liquids, the cohesive force between molecules is predominant over molecular momentum transfer.
- With increasing temperature, the cohesive force between molecules decreasing so the viscosity of liquids decreasing with increasing temperature.
Surface Tension
- Reason: Cohesive force between the molecules.
- Definition: Force required to maintain unit length of the film in equilibrium, i.e., force per unit length.
- Unit: (N/m)
- Due to surface tension
- Increase in the internal pressure of droplet.
- The tendency of the liquid droplet to attain minimum surface area at a given volume, only for this reason, shape of droplet is “Sphere”.
NOTE:
Minimum surface area at a given volume = surface area of the sphere.
- Work done, in shifting of wire through a distance of (Δx) :
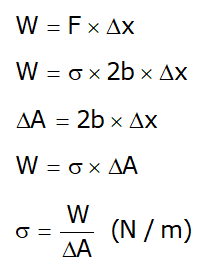
- From this, surface tension can also be defined as Work was done per unit change in the surface area of the liquid.
The dependency of Surface Tension
Temperature
- If temperature increases, the cohesive force decreases, and this will result in a decrease in surface tension.
- Surface tension becomes zero at the “critical point of temperature”.
Excess Pressure:
- Due to surface tension pressure inside the bubble become higher than the external atmospheric pressure.
- Excess Pressure is the difference between internal pressure (pi) and external pressure (p0).
- For soap bubble:
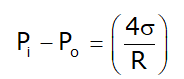
- For Liquid droplet:
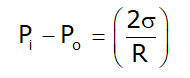
Where σ is surface tension and R is the radius of curvature for bubble or droplets.
Capillary Effect
- Reason: Cohesive force or surface tension and Adhesive forces. (Both force responsible for Capillary effect)
- The free surface having a Concave or convex top, inside the capillaries is called meniscus.
- The rise or fall of liquid inside the tube is due to contact angle b/w liquid surface and capillary tube.
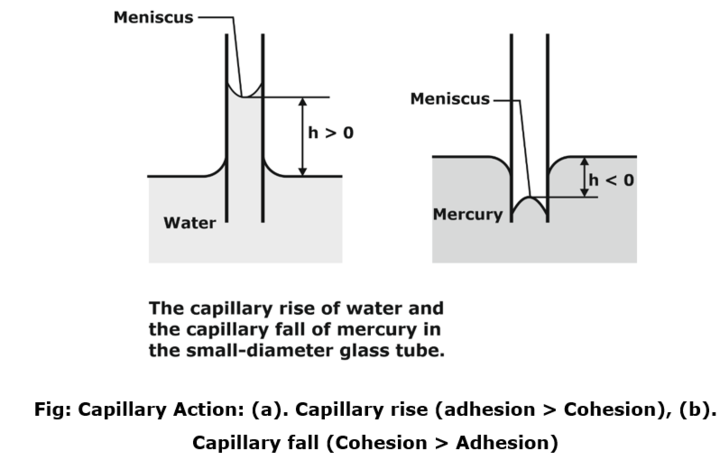
NOTE:
If ![]() then:
then:
- Level of liquid inside the tube is rise.
- Liquid is known as Wetting liquid
- In this case:
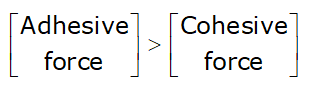
If ![]() then:
then:
- Level of liquid fall inside the tube
- liquid is known as Non-wetting liquid
- In this case:
Φ Angle between tangent to the liquid surface and solid surface at contact point.
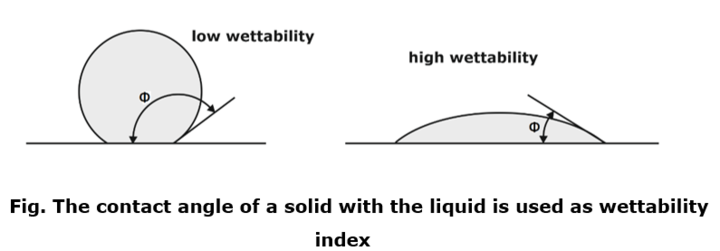
Height of capillary rise
- By equilibrium:
- Upward force = Downward force (Surface tension= Weight of water)
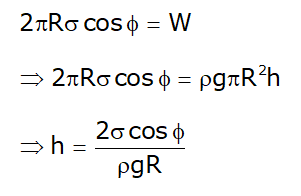
Observations
- For water –glass interface
 So cosΦ=1 this results in:
So cosΦ=1 this results in: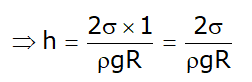
- Height of capillary rise is a function of:
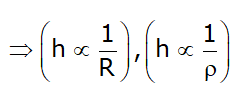
- If the diameter of the tube > 1 cm than the Capillary effect negligible
Vapour Pressure and Cavitation:
- Saturation Temperature
- For a given pressure, the temperature at which a pure substance changes phase is known as saturation temperature.
- Saturation Pressure:
- At a given temperature, the pressure at which a pure substance changes phase i known as saturation temperature.
Example: at 1 atm pressure (const. pressure) saturation temperature is 100°C and at a constant temp. 100°C saturation pressure for water is 1 atm.
Vapour Pressure
- For liquid, the pressure exerted by its vapour, in phase equilibrium with its liquid at a given temperature is known as vapour pressure.
- Vapour pressure increases with an increase in temperature as the rate of molecules escaping liquid surface increases.
- The temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere – boiling occur.
Cavitation
- Cavitation is a phenomenon which occurs in a liquid flow system.
- If liquid undergoes a pressure below its vapour pressure during flow than sudden vaporization takes place and vapour bubbles formed.
- Vapour bubbles collapse as they move from the low-pressure region to a high-pressure region, generating highly destructive pressure waves.
- Cavitation can also occur if a liquid contains dissolved air or other gases, (Reason-Solubility decreases with decreasing pressure and their bubbles form).
- The risk of cavitation is greater at a higher temperature.
Example: Given a flow system (water) and Temperature is 36°C. Find the minimum pressure to avoid cavitation?
Solution: Minimum pressure to avoid cavitation is equal to vapour pressure of that liquid at given temperature for water![]()
Note:
- Partial pressure is the pressure exerted by a component in a mixture of gases.
- For a pure substance, vapour pressure and saturation pressure, both are equal.
- If the external pressure is equal to or less than the vapour pressure, boiling of liquid will start no matter how much temperature.
Bulk Modulus of Elasticity
- The compressibility of liquid is measured by the bulk modulus of elasticity.
- More is the bulk modulus less is the compressibility.
- Compressibility is reciprocal of Bulk modulus.
- Bulk modulus is represented as the compressive stress per unit volumetric strain.
- Bulk modulus (k):

- K → always positive or is a positive quantity having unit of pressure
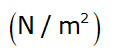 .
. - Truly incompressible substance: means
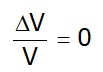 .
.
So, K (bulk modulus) = ∞
Note:
K increase means Resistance to further compression increases.
- For liquid K increases with decreases in temperature: with a decrease in temperature cohesive force between molecules increases, which results in higher resistance to further compression.
- For gases, K increases with increases in temperature: With an increase in temperature, a collision between gas-particle increases and results in higher internal pressure so the resistance to further compression increases.
No comments:
Post a Comment
Knowing brings controversy